
The aim is to get the product on store shelves.Continue reading

The WHO predicts that by 2050, the leading cause of death could be infections caused by microorganisms resistant to conventional antibiotics. To fight them, new types of compounds with specific ways of responding are needed. Researchers from the HUN-REN Research Center for Natural Sciences (HUN-REN TTK) have now reported a possible promising direction in a study published in the prestigious journal Nature Communications.
It is no coincidence that superbacteria resistant to conventional antibiotics have become the focus of researchers’ studies: the WHO’s first comprehensive analysis in 2020, already showed that resistant bacteria caused 1.27 million deaths in 2019, and unfortunately this number is multiplying year by year. One way to fight superbacteria is to use so-called phage therapy, where they are killed by a virus. Research along these lines is being carried out, for instance, at the Szeged Biological Research Center. Another promising option is the development of antibiotics with completely new mechanisms of responding.
“The small-cell systems of bacteria are incredibly adaptive, sometimes building up an entire colony overnight. In fact, recent research suggests that some bacteria secrete nanometer-sized ‘alarm balls’ of themselves when they die, passing on the knowledge they need to survive to their fellow bacteria. This incredible adaptability also suggests that superbacteria need to be fought from several directions,” said Tamás Beke-Somfai, head of the Biomolecular Self-assembly Research Group at the HUN-REN Faculty of Technology.
“In our own body we have naturally based peptides, we call them antimicrobial peptides. We have designed supramolecular structures – in fact a new antibiotic – that combine the bactericidal mechanisms of these natural peptides with the favorable biostability of non-natural compounds,” said the Hungarian researcher.
Until now, researchers have not really been able to observe how the peptides “attack,” but the Hungarians researchers have now succeeded in tracing how they are arranged into supramolecular structures on the surface of bacterial cells using transmission and cryo-electron microscopy.
“The recordings show that the lamellae that form penetrate the cell wall (like knives) and penetrate the inside of the bacterium. The puncturing of the cell wall causes a leak, which eventually leads to the death of the bacterium. Image analysis has also shown that surprisingly few of the lamellae that penetrate the bacterial cell are sufficient to cause cell wall damage. In addition, the tests show that the peptides inhibit bacterial growth at very low concentrations,” summarized the research team leader.
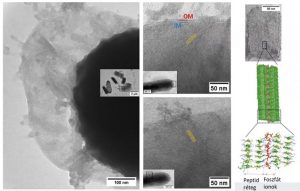
Peptide lamellae (yellow stripes) form on the surface of bacteria and disrupt the cell wall (OM and IM) as they grow into it (left). Molecular structure of peptide-based supramolecules (right). Source: hun-ren.hu
The microscopic images and molecular dynamics simulations confirmed that the formation of peptide-based lamellae is based on the interaction with phosphate groups: even in the presence of simple inorganic phosphate ions, banded lamellar morphologies are formed in which each band is composed of two or more peptide layers, linked by phosphate ions and stabilized by hydrogen bonds.
The work of the HUN-REN TTK researchers is innovative in several ways:
Firstly, they have successfully designed a peptide-based antibiotic that is activated in-situ by binding specifically to bacterial cells, and secondly, they are the first to gain direct visual insights into the function of bactericidal peptides.
Via HUN-REN, Featured image: Pixabay