
Resistant bacteria caused 1.27 million deaths in 2019, and unfortunately this number is multiplying year by year.Continue reading

Hungarian researchers have created a unique superbacteria map, detailing the global presence and spread of one of the most dangerous hospital-acquired bacteria. The quality of the research and the importance of the topic is reflected in the publication of the team’s findings in Cell, one of the world’s most prestigious life sciences journals, HUN-REN Research Center writes.
The results of the research, led by the National Laboratory of Biotechnology at the HUN-REN Biological Research Center, Szeged, could open a new chapter in the fight against antibiotic-resistant hospital-acquired infections. Researchers are using the map to develop a bacteriophage-based treatment that targets and kills the most prevalent superbacteria as a promising alternative to ineffective antibiotics.
The research teams led by Bálint Kintses and Balázs Papp, in collaboration with the National Public Health Center (NNGYK), the National Laboratory for Health Security and several healthcare institutions in five Eastern European countries,
have mapped the prevalence of one of the most dangerous hospital pathogens (Acinetobacter baumannii) in Eastern Europe and worldwide, and the patterns of spread of each variant.
Collected systematically, this information is invaluable for epidemiological forecasting and drug development.
The superbacterial map is based on a detailed analysis of the bacterial genome, providing accurate information on the diversity of the superbacterial species studied. There can be hundreds of variants of antibiotic-resistant bacteria within a given species. This poses a major obstacle to bacteriophage therapy, that is effective against multi-drug resistant pathogens and is one of the most promising alternatives to conventional antibiotics, but also one of the most challenging in terms of drug development.
Bacteriophages are ‘good’ viruses that infect and kill only bacteria, leaving human cells intact. However, phages are highly specific: within each bacterial species, a phage must be found for each variant. Once an infection has developed, there is a time frame of up to one to two weeks to reverse the condition. Unfortunately, the short time frame does not allow for the identification of the exact bacterial variant, the discovery of the bacteriophage that is effective and the production of a phage formulation that can be used for therapeutic purposes, the research center writes.
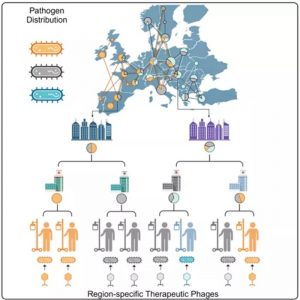
Using an unprecedented world map, the researchers have discovered that although there is a huge diversity within species, only a few variants predominate in a region.
If at least 8-10 different bacteriophage formulations tailored to a particular region were available, 80 percent of hospital-acquired infections caused by that bacterium could be treated.
Source: hun-ren.hu
In addition, if we know which countries have roughly the same variants of the same bacteria infecting hospitalized patients, then global collaboration could lead to more effective clinical trials of targeted phage cocktails.
The superbacteria map gives hope that phage therapy could become an integral part of the state-of-the-art toolkit for personalized patient care in the not too distant future – just as antibiotics were at the turn of the last century.
Via HUN-REN, Featured image: Pexels